TECHNOLOGY
A Targeted Approach to Smarter Drug Delivery
CuboPharm’s patented cubosome platform is a lipid-based nanoparticle system designed to overcome key challenges in drug delivery — particularly for compounds with poor solubility or high first-pass metabolism.
Our sublingual, orally disintegrating formulation enables direct systemic absorption, bypassing the liver and minimizing the formation of toxic metabolites.
The result is enhanced bioavailability, a faster onset of action, and a significant reduction in side effects.
Innovation and mechanism of action Rosuvastatin Orally Disintegrating Tablet (ODT)
A novel sublingual formulation utilising amphiphiles that self-assemble into cubosomes upon
contact with saliva. These bio adhesive nanostructures form a gel-like matrix in the
sublingual cavity, enabling sustained API release, prolonged mucosal contact, and effective
absorption – while avoiding first-pass hepatic metabolism.
BENEFITS
- Improves absorption of poorly soluble and highly metabolised drugs
- Avoids first-pass metabolism
- Reduces toxic metabolite formation
- Enhances safety and patient tolerability
- Convenient and patient friendly. No water required
- Scalable, GMP-ready, and protected by global patents
- Suitable for delivery of molecules up to 10 kDa
– Applicable to a broad range of APIs
– Supports the incorporation of diverse active compounds
– Can accommodate diverse APIs, including hydrophilic and lipophilic molecules
The following APIs have been tested at the synchrotron at various concentrations:
- Niacin
- Morphine Sulfate
- Rosuvastatin
- Atorvastatin
- Simvastatin
- Ibuprofen Lysine
- Oxycontin
- Adrenaline
- Dalteparin
- Enoxaparin
RESEARCH
SUBLINGUAL ROSUVASTATIN
Cardiovascular disease (CVD) is the leading cause of death worldwide, with elevated low-density lipoprotein cholesterol (LDL-C) being a major contributor. Statins — such as simvastatin, atorvastatin, and rosuvastatin — are the gold standard for lowering LDL-C and reducing cardiovascular risk. However, up to 30% of patients experience statin intolerance, often presenting as muscle pain, weakness, or other side effects, leading to poor adherence and inadequate treatment outcomes.
In response to this challenge, a long-term research and clinical initiative was launched in 2012 to develop alternative therapies for statin-intolerant patients. The focus was on creating a more tolerable delivery method for rosuvastatin that bypasses the gastrointestinal tract, where many side effects originate.
Through an innovative approach involving cutting-edge synchrotron technology and interdisciplinary collaboration, including with
Monash Institute of Pharmaceutical Sciences (MIPS) researchers identified a lipophilic “envelope” that enhances transdermal and submucosal absorption of rosuvastatin. This led to the development of a sublingual
rosuvastatin oral disintegrating tablet (ODT), enabling the drug to be absorbed directly through the mucosal tissues under the tongue.
Clinical trials demonstrated that this sublingual formulation not only maintains the lipid-lowering efficacy of traditional statins but also reduces side effects by over 90%. This advancement provides a well-tolerated, cost-effective alternative for patients who are unable to use conventional statins, ultimately supporting better long-term cardiovascular outcomes.
A clinical trial was conducted to assess the efficacy and tolerability of the resulting ODT in patients.
Clinical Effects of Sublingually Administered Rosuvastatin in Subjects Who Are Statin Intolerant (SARSI)
In this phase 2, 6-week double blind, randomised, crossover clinical trial involving 24 patients, the novel rosuvastatin ODT demonstrated significant clinical efficacy:
- 95% patient tolerability
- 42% reduction in LDL-C
- 29% reduction in total cholesterol
The novel rosuvastatin ODT effectively reduces LDL-C and total cholesterol with high patient tolerability. The formulation was approved by the Therapeutic Goods Administration (TGA) in April 2020 and is available through the Special Access Scheme for eligible patients.
The success of this trial opens the door to further exploration of alternative therapeutic drug applications using this delivery system.
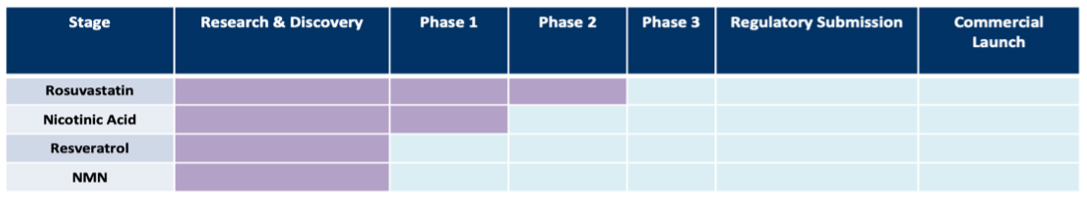
PROJECT PIPELINE
DRUG DEVELOPMENT PIPELINE TIMELINE
PATENT LIST
COLLABORATIONS
Monash Institute of Pharmaceutical Sciences (MIPS), CSIRO, AusIndustry, Australian Synchrotron, Aspen Pharmacare, Medicines, Manufacturing, Innovative Centre (MMIC)





